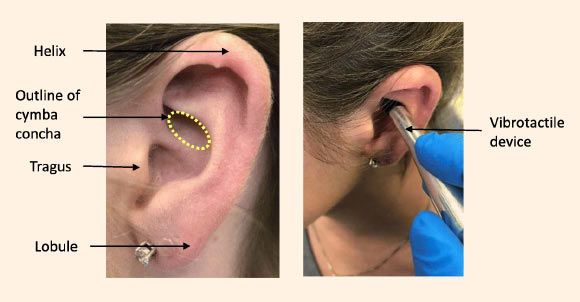
Researchers at Harvard Medical School recently discovered that. This non-invasive vagus nerve stimulation device has electrodes which are attached to the tragus section of the outer ear.
 Vagus Nerve Stimulation Via The Outer Ear Takes Center Stage Psychology Today
Vagus Nerve Stimulation Via The Outer Ear Takes Center Stage Psychology Today
VNS is an FDA-approved therapy for the treatment of both depression and epilepsy but it is limited to the management of more severe intervention-resistant cases as a second or third-line treatment option due to perioperative risks involved with device.

Transcutaneous vns device. More recently non-implantable VNS devices also referred to as n-VNS or transcutaneous VNS t-VNS have been developed to treat migraine and cluster headaches. Transcutaneous vagus nerve stimulation tVNS is a newly developed method which intends to overcome the disadvantage of surgical implantation of the stimulation device. VNS devices are currently being investigated for stroke rehabilitation treatment of chronic heart failure and rheumatoid arthritis.
Previous tVNS neuroimaging studies revealed brain deactivation patterns that involved multiple areas implicated in tinnitus generation and perception. 7 hours agoThe spinal cord stimulation SCS devices segment of the global neurostimulation devices market is expected to grow from 23 billion in 2021 to 27 billion in 2026 at a CAGR of 34 for the. Non-Invasive vagus nerve stimulation gammaCore gammaCore is a simple-to-use handheld medical device that enables patients to self-administer discrete doses of non-invasive vagus nerve stimulation nVNS therapy.
A vagus nerve stimulation VNS device that hooks onto the ear shows promise as a new non-invasive treatment for indigestion. 7 hours agoNeurostimulation technologiesdevices include spinal cord stimulation deep brain stimulation sacral nerve stimulation vagus nerve stimulation hypoglossal nerve stimulation transcutaneous. In this method an electrical pulse generated by a device placed in one or both ears stimulates the auricular branch of.
We propose the use of a physiologically-enhanced transcutaneous VNS tVNS as a low risk non-invasive and inexpensive alternative. NVNS therapy has been shown to be a safe and effective treatment option. The tVNS device is designed to stimulate the auricular branch of the vagus nerve via a bipolar electrode attached to the skin of the left ear conch.
GammaCore is a CE-mark approved non-medicinal treatment for adults who get cluster headache. An example of this type of device is gammaCore-S ElectroCore LLC which is a noninvasive handheld prescription device intended to deliver transcutaneous vagus. Transcutaneous VNS tVNS has been gaining popularity as a noninvasive alternative to VNS.
Several animal and human trials have shown promising. Transcutaneous auricular vagus nerve stimulation t-VNS is an alternative procedure for vagus nerve stimulation that does not require surgical intervention. Several studies have illustrated that transcutaneous vagus nerve stimulation tVNS can elicit therapeutic effects that are similar to those produced by its invasive counterpart vagus nerve stimulation VNS.
Whereas invasive VNS is an established neuromodulatory treatment in drug-resistant epilepsy transcutaneous VNS tVNS of the auricular branch of the vagus nerve is suggested to be an alternative access path to the same neuronal network without invasiveness. The stimulating current is administered using a transcutaneous electrical. Recently devices to noninvasively affect vagus nerve functioning have been developed with the aim of achieving similar effects without the drawbacks of a surgical procedure and continuous stimulation.
Vagal nerve stimulation VNS FDA-approved for MDD modulates brain circuitry implicated in moodanxiety and autonomic regulation however it is implanted and thus invasive. At different areas of the vagus nerve to treat conditions like obesity.