Your blood cells will be sent to a manufacturing center to make your YESCARTA. Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements and must have on-site immediate access to a minimum of 2 doses of tocilizumab for each patient for infusion within 2 hours after YESCARTA.
Manufacturing Challenge Leads To Death In Car T Trial
We are also continuing to expand our manufacturing operations outside the US.
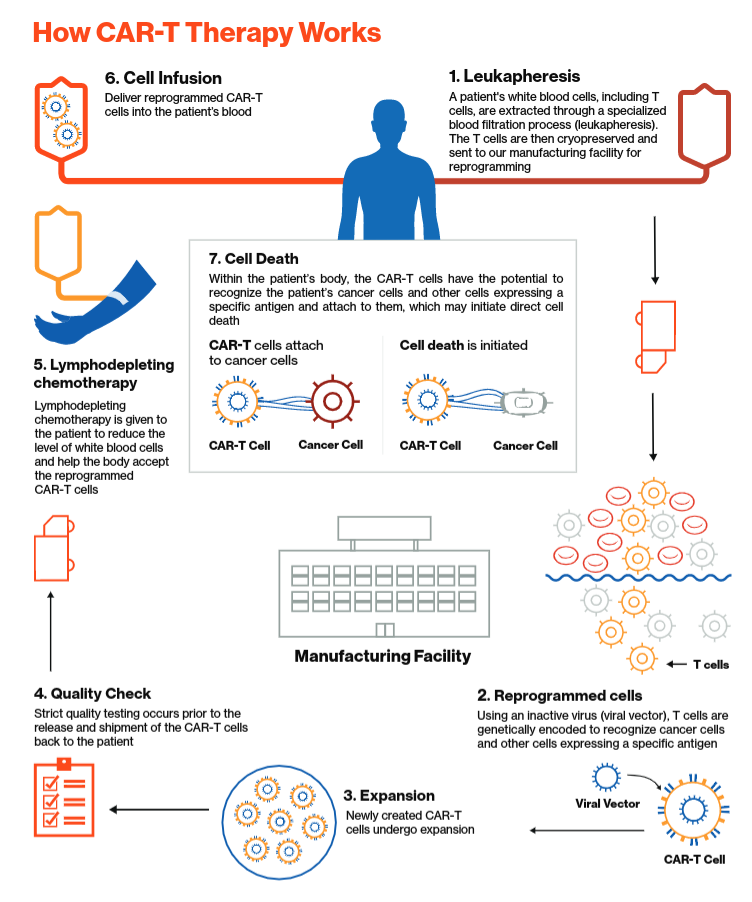
Yescarta manufacturing process. This is mainly due to the personalised nature of CAR T therapies. The manufacturing difference range from taking the starting material direct from the patient and the uniqueness of each batch to the necessity for continuous processing from apheresis to final product and quality systems to cover the entire end-to-end process. MANUFACTURING PROCESS LEUKAPHERESIS 3 to 4 hours7.
Your blood cells will be sent to a manufacturing center to make your YESCARTA. Apheresis device selection and. Gileads Kite builds cell therapy manufacturing quickly even as Yescarta sales grow slowly Gileads Kite Pharma which is already building a cell therapy manufacturing facility in.
These operations are manually intensive ergonomically chal- lenging and inherently risky for large scale manufacturing. Before you get YESCARTA you will get 3 days of chemotherapy to prepare your body. Your blood cells will be sent to a manufacturing center to make your YESCARTA.
YESCARTA is shipped in a vapor phase liquid nitrogen dry shipper dewar to the. A number of CAR T products are manufactured using manual processing which is labour intensive difficult to scale and prone to high failure rates 45. Since YESCARTA is made from your own white blood cells your blood will be collected by a process called leukapheresis loo-kah-fur-ee-sis which will concentrate your white blood cells.
We are busy with the global expansion of Yescarta and are currently taking the existing process and transferring it to additional manufacturing sites in appropriate regions. Manufacturing process definition of starting material potency assay for release testing and release specification Replication -Competent Retrovirus testing te sting and release strategy for cell banks sterility testing comparability following manufacturing changes stability programme and shelf life. At least 1-7 years 6 weeks.
To scale up manufacturing processes have to move from being open and manual to closed and automated reducing contamination and risk increasing product consistency and efficiency and adding traceability throughout the process to ensure chain of custody. Because of the risk of CRS and neurologic toxicities YESCARTA is available only through a restricted program called the YESCARTA and TECARTUS REMS Program which requires that. II0008 BIa2c - Changes in the manufacturing process of the AS - The change refers to a - substance in the manufacture of a biologicalimmunological substance which may have a significant impact on the medicinal product and is not related to a protocol 19092019 na II0007 BIIb3c - Change in the manufacturing process of the finished or intermediate product - The product is 2507.
The manufacturing process is YESCARTA is cryopreserved and stored at not greater than -150C. However research and development has still not created a mature fully understood process and thus product. A single infusion of YESCARTA is administered to the.
Development and clinical production of CAR-T therapies relies heavily upon the use of open processing steps that are performed in ISO 5Grade A biological safety cabinet BSC hoods to reduce the risk of advantageous agent contamination of pro- cess materials. Gilead Sciences which produces Yescarta the second CAR-T therapy approved in the US has also been expanding rapidly including opening a long-awaited 117000-square-foot plant at. Patient T cells are extracted and then frozen to ship to a manufacturing facility where the cells are genetically engineered to seek out cancers expressing certain proteins.
For example we just recently announced plans to establish a new manufacturing facility in Europe at Schiphol Airport just outside Amsterdam. The patients T cells are isolated and engineered ex vivo at a state-of-the-art. When your YESCARTA is ready your.
Yescarta of KITE Pharma the first CAR T cell products have been approved. The goal of apheresis in the clinical manufacturing context is to have a consistent robust process that is optimal for the therapeutic product without sacrificing the safety and comfort of the donor. The souped-up immune cells are then refrozen shipped back to a medical facility and reinfused into the patient.
Meanwhile information flow needs differ for cell therapies as they require a chain of custody throughout the process and timing and. Since YESCARTA is made from your own white blood cells your blood will be collected by a process called leukapheresis loo-kah-fur-ee-sis which will concentrate your white blood cells. Ideally the process can be used to lessen requirements on the starting material by imposing separation protocols during collection that minimize downstream handling.
Lymphocytes are collected from the patient CELL MANUFACTURING Median of 16 days3. Since YESCARTA is made from your own white blood cells your blood will be collected by a process called leukapheresis loo-kah-fur-ee-sis which will concentrate your white blood cells.
