Novartis Pharmaceuticals Corporation researches develops manufactures and markets innovative medicines aimed at improving patients lives. Comparison of Secukinumab Versus Adalimumab Efficacy on Skin Outcomes in Psoriatic Arthritis.
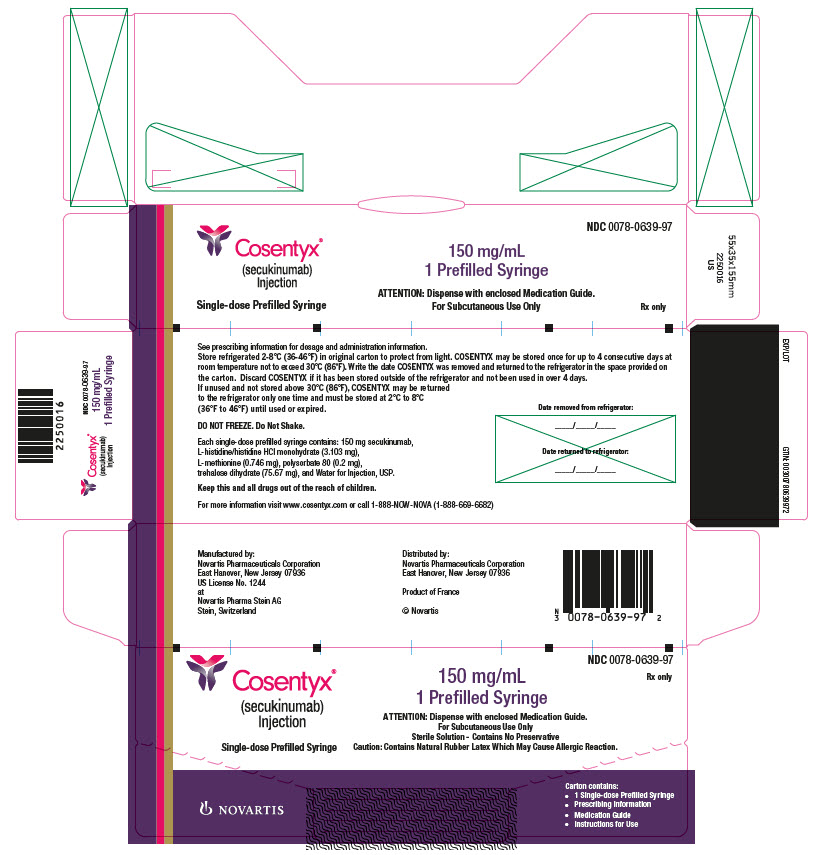 Cosentyx Information Side Effects Warnings And Recalls
Cosentyx Information Side Effects Warnings And Recalls
7 2015 PRNewswire -- Novartis announced two-year data for Cosentyx secukinumab from an observational sub-analysis of a.

Who manufactures cosentyx. Food and Drug Administration FDA has approved a label update for Taltz ixekizumab injection 80 mgmL to include data in psoriasis involving the genital area. Novartis Reports Cosentyx Data. Mar 21 2015 0704 ET.
EAST HANOVER NJ Jan. Looking forward communities across the globe are relying on the pharmaceutical industry to create solutions and many of the companies in the top 10 are involved in the race to produce treatment or prevention measures for the Covid-19 disease. 1 Taltz is the first and only treatment approved by the FDA for moderate-to-severe plaque psoriasis that.
Secukinumab Cosentyx for the treatment of active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated C-reactive protein andor magnetic resonance imaging evidence in adults who have responded inadequately to. In the same quarter Novartis booked 937 million in global sales of Cosentyx suggesting AbbVie and Boehringer Ingelheim have plenty of room to. 15 2016 PRNewswire -- Novartis announced today that the US Food and Drug Administration FDA has approved Cosentyx secukinumab for two new indications - the treatment of adult patients with active ankylosing spondylitis AS and active psoriatic arthritis PsA.
Novartis which manufactures secukinumab Cosentyx has been a corporate sponsor of the Global Healthy Living Foundation. INDIANAPOLIS May 22 2018 PRNewswire -- Eli Lilly and Company NYSE. This includes Crohns disease ulcerative colitis rheumatoid arthritis ankylosing spondylitis psoriasis psoriatic arthritis and Behçets disease.
Infliximab a chimeric monoclonal antibody sold under the brand name Remicade among others is a medication used to treat a number of autoimmune diseases. EAST HANOVER NJ Jan. Enbrel etanercept is a prescription medication used to treat five chronic diseases including moderate to severe rheumatoid arthritis RA psoriatic arthritis PsA moderate to severe plaque psoriasis PsO ankylosing spondylitis AS and moderate to severe polyarticular juvenile idiopathic arthritis JIA.
Novartis is a global healthcare company based in Switzerland that provides solutions to address the evolving needs of patients worldwide. 15 2016 PRNewswire -- Novartis announced today that the US Food and Drug Administration FDA has approved Cosentyx secukinumab for two new indications -. 52 Week Results from the Exceed Study.
Lantheus is the parent company of Lantheus Medical Imaging which manufactures diagnostic imaging agents and products. EAST HANOVER NJ Nov. For active immunization against influenza for individuals 3 years of age or older and to include the data from pediatric clinical studies with revision of the prescribing information.
EAST HANOVER NJ March 21 2015 PRNewswire -- Novartis today announced new two-year results demonstrating sustained efficacy with Cosentyx. LLY announced today that the US. Abbvie Inc which manufactures adalimumab Humira and Novartis Pharmaceuticals which manufactures secukinumab Cosentyx are corporate sponsors of the Global Healthy Living Foundation.
About Cosentyx Cosentyx is the first and only fully-human biologic that directly inhibits IL-17A a cornerstone cytokine involved in the inflammation and development of PsO PsA and AS 8-11. Please see Indications and Important Safety Information. Sources Cosentyx FDA Approval History.
Sources Gottlieb AB et al. It is given by slow injection into a vein typically at six- to eight-week intervals. The Covid-19 pandemic which was first discovered at the end of 2019 has had an unprecedented impact on the world.
